Automated Visual Evaluation (AVE)
AVE is an innovative artificial intelligence tool developed that consists of a machine learning (ML) classifier and a decision-making algorithm, which identifies cervical pre-cancer from a colposcopic image. AVE “learns” to identify patterns of high-grade pre-cancer from cervical images to differentiate CIN lesions from normal tissue. Retrospective preliminary data shows that AVE may have higher detection of CIN2+ than either cervicography or conventional cytology screening, and similar performance to HPV testing or colposcopy for detection of CIN2+.
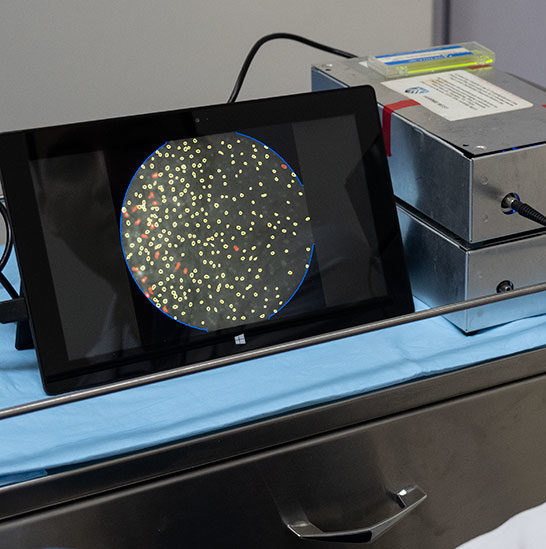 HRME: High-Resolution Micro Endoscopy
HRME: High-Resolution Micro Endoscopy
The HRME imaging system is intended to be a low-cost, innovative technique that allows real-time, point-of-care detection of high-grade precancerous cervical lesions without a biopsy being performed. As part of Rice University and MD Anderson led study, BHI enrolled more than 1800 women in this project.

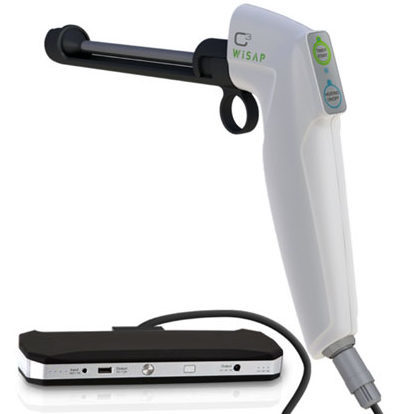
LMIC-Adapted Cryopen®
Instead of using cryogenic gas like conventional cryotherapy, the Cryopen® (CryoPen Inc., Southlake, TX) utilizes electricity or power from a car battery to freeze cervical precancer lesions.

 Thermal Ablation
Thermal Ablation
 HRME: High-Resolution Micro Endoscopy
HRME: High-Resolution Micro Endoscopy